
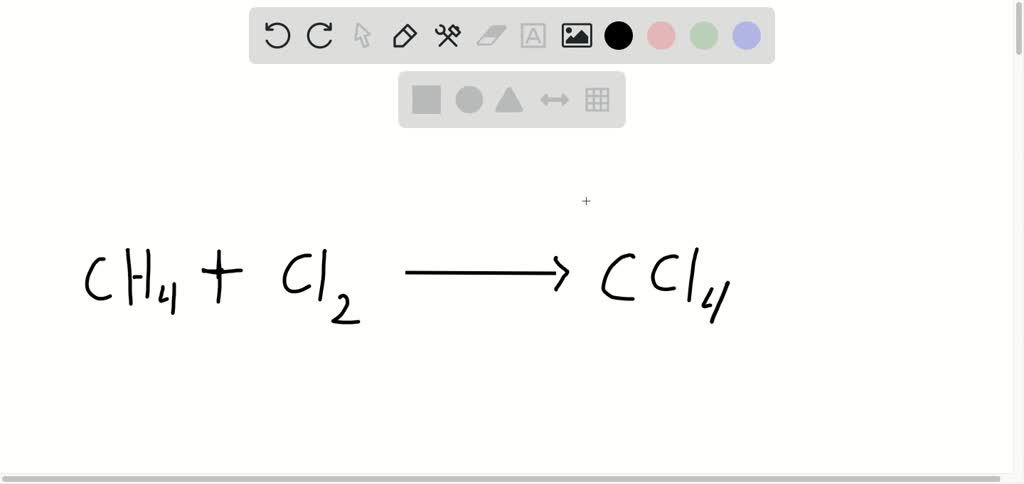
The transition elements and inner transition elements also do not follow the octet rule:įor more complicated molecules and polyatomic ions, it is helpful to follow the step-by-step procedure outlined here: Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CCl 4 (carbon tetrachloride) and silicon in SiH 4 (silane). For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons) this is especially true of the nonmetals of the second period of the periodic table (C, N, O, and F). The tendency of main group atoms to form enough bonds to obtain eight valence electrons is known as the octet rule. This allows each halogen atom to have a noble gas electron configuration. The other halogen molecules (F 2, Br 2, and I 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. Each Cl atom interacts with eight valence electrons: the six in the lone pairs and the two in the single bond. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:Ī single shared pair of electrons is called a single bond. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions. The total number of electrons does not change. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:įigure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.


 0 kommentar(er)
0 kommentar(er)
